Current State and Opportunities with Long-acting Injectables: Industry Perspectives from the Innovation and Quality Consortium “Long-Acting Injectables” Working Group

Long-acting injectable (LAI) formulations can provide several advantages over the more traditional oral formulation as drug product opportunities. LAI formulations can achieve sustained drug release for extended periods of time, which results in less frequent dosing requirements leading to higher patient adherence and more optimal therapeutic outcomes. This review article will provide an industry perspective on the development and associated challenges of long-acting injectable formulations. The LAIs described herein include polymer-based formulations, oil-based formulations, and crystalline drug suspensions. The review discusses manufacturing processes, including quality controls, considerations of the Active Pharmaceutical Ingredient (API), biopharmaceutical properties and clinical requirements pertaining to LAI technology selection, and characterization of LAIs through in vitro, in vivo and in silico approaches. Lastly, the article includes a discussion around the current lack of suitable compendial and biorelevant in vitro models for the evaluation of LAIs and its subsequent impact on LAI product development and approval.
This is a preview of subscription content, log in via an institution to check access.
Subscribe and save
Springer+ Basic
€32.70 /Month
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Buy Now
Price includes VAT (France)
Instant access to the full article PDF.
Rent this article via DeepDyve

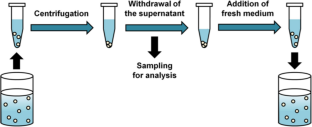
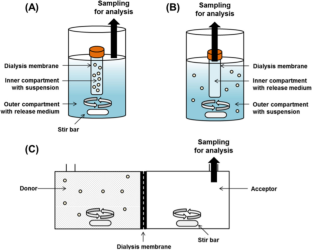
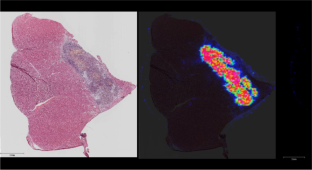

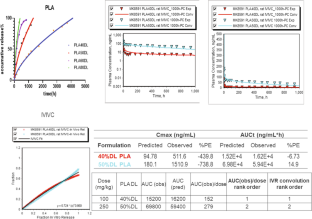
Similar content being viewed by others

Long-Acting Injectable Aqueous Suspensions—Summary From an AAPS Workshop
Article 28 April 2023

Inspirational chemistry of bioabsorbable long-acting injectables
Article 21 August 2022
A New Level A Type IVIVC for the Rational Design of Clinical Trials Toward Regulatory Approval of Generic Polymeric Long-Acting Injectables
Article 27 June 2016
Explore related subjects
References
- Li W, Tang J, Lee D, Tice TR, Schwendeman SP, Prausnitz MR. Clinical translation of long-acting drug delivery formulations. Nature Rev Mater. 2022.
- Shi Y, Lu A, Wang X, Belhadj Z, Wang J, Zhang Q. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharm Sin B. 2021;11(8):2396–415. ArticleCASPubMedPubMed CentralGoogle Scholar
- Pizzorno A, Padey B, Terrier O, Rosa-Calatrava M. Drug Repurposing Approaches for the Treatment of Influenza Viral Infection: Reviving Old Drugs to Fight Against a Long-Lived Enemy. Front Immunol. 2019;10:531. ArticleCASPubMedPubMed CentralGoogle Scholar
- Selmin F, Musazzi UM, Magri G, Rocco P, Cilurzo F, Minghetti P. Regulatory aspects and quality controls of polymer-based parenteral long-acting drug products: the challenge of approving copies. Drug Discov Today. 2020;25:321–9. ArticlePubMedGoogle Scholar
- Bassand C, Villois A, Gianola L, Laue G, Ramazani F, Riebesehl B, Sanchez-Felix M, Sedo K, Ullrich T, Duvnjak Romic M. Smart design of patient-centric long-acting products: from preclinical to marketed pipeline trends and opportunities. Expert Opin Drug Deliv. 2022:1–19.
- Burness CB, Dhillon S, Keam SJ. Lanreotide autogel(®): a review of its use in the treatment of patients with acromegaly. Drugs. 2014;74(14):1673–91. ArticleCASPubMedGoogle Scholar
- Schwendeman SP, Shah RB, Bailey BA, Schwendeman AS. Injectable controlled release depots for large molecules. Journal of controlled release : official journal of the Controlled Release Society. 2014;190:240–53. ArticleCASPubMedGoogle Scholar
- Kamaly N, Yameen B, Wu J, Farokhzad OC. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem Rev. 2016;116(4):2602–63. ArticleCASPubMedPubMed CentralGoogle Scholar
- Nkanga CI, Fisch A, Rad-Malekshahi M, Romic MD, Kittel B, Ullrich T, Wang J, Krause RWM, Adler S, Lammers T, Hennink WE, Ramazani F. Clinically established biodegradable long acting injectables: An industry perspective. Adv Drug Deliv Rev. 2020;167:19–46. ArticleCASPubMedGoogle Scholar
- Ford Versypt AN, Pack DW, Braatz RD. Mathematical modeling of drug delivery from autocatalytically degradable PLGA microspheres–a review. Journal of controlled release : official journal of the Controlled Release Society. 2013;165(1):29–37. ArticleCASPubMedGoogle Scholar
- Zolnik BS, Burgess DJ. Effect of acidic pH on PLGA microsphere degradation and release. J Control Release. 2007;122(3):338–44. ArticleCASPubMedGoogle Scholar
- Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. J Control Release. 2006;112(3):293–300. ArticleCASPubMedGoogle Scholar
- Wang J, Helder L, Shao J, Jansen JA, Yang M, Yang F. Encapsulation and release of doxycycline from electrospray-generated PLGA microspheres: Effect of polymer end groups. Int J Pharm. 2019;564:1–9. ArticleCASPubMedGoogle Scholar
- Félix Lanao RP, Leeuwenburgh SC, Wolke JG, Jansen JA. In vitro degradation rate of apatitic calcium phosphate cement with incorporated PLGA microspheres. Acta Biomater. 2011;7(9):3459–68. ArticlePubMedGoogle Scholar
- Christen MO, Vercesi F. Polycaprolactone: How a Well-Known and Futuristic Polymer Has Become an Innovative Collagen-Stimulator in Esthetics. Clin Cosmet Investig Dermatol. 2020;13:31–48. ArticleCASPubMedPubMed CentralGoogle Scholar
- Woodruff MA, Hutmacher DW. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog Polym Sci. 2010;35(10):1217–56. ArticleCASGoogle Scholar
- Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Adv Drug Deliv Rev. 2002;54(7):889–910. ArticleCASPubMedGoogle Scholar
- Halpern V, Stalter RM, Owen DH, Dorflinger LJ, Lendvay A, Rademacher KH. Towards the development of a longer-acting injectable contraceptive: past research and current trends. Contraception. 2015;92(1):3–9. ArticleCASPubMedGoogle Scholar
- Tamilvanan S, Venkateshan N, Ludwig A. The potential of lipid- and polymer-based drug delivery carriers for eradicating biofilm consortia on device-related nosocomial infections. J Control Release. 2008;128(1):2–22. ArticleCASPubMedGoogle Scholar
- Shi Y, Lu A, Wang X, Belhadj Z, Wang J, Zhang Q. A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives. Acta Pharmaceutica Sinica B. 2021;11(8):2396–415. ArticleCASPubMedPubMed CentralGoogle Scholar
- Yang S, Hu M, Liu W, Hou N, Yin K, Shen C, Shang Q. Fabrication of PLGA in situ forming implants and study on their correlation of in vitro release profiles with in vivo performances. J Biomater Sci Polym Ed. 2021;32(8):994–1008. ArticleCASPubMedGoogle Scholar
- Park K, Skidmore S, Hadar J, Garner J, Park H, Otte A, Soh BK, Yoon G, Yu D, Yun Y, Lee BK, Jiang X, Wang Y. Injectable, long-acting PLGA formulations: Analyzing PLGA and understanding microparticle formation. J Control Release. 2019;304:125–34. ArticleCASPubMedGoogle Scholar
- Chaudhary K, Patel MM, Mehta PJ. Long-Acting Injectables: Current Perspectives and Future Promise. Crit Rev Ther Drug Carrier Syst. 2019;36(2):137–81. ArticlePubMedGoogle Scholar
- Kanwar N, Sinha VR. In Situ Forming Depot as Sustained-Release Drug Delivery Systems. Crit Rev Ther Drug Carrier Syst. 2019;36(2):93–136. ArticlePubMedGoogle Scholar
- Malik K, Singh I, Nagpal M, Arora S. Atrigel: A potential parenteral controlled drug delivery system. Der Pharmacia Sinica. 2010;1:74–81. CASGoogle Scholar
- Ss R, Gs B, B. SR. A REVIEW ON: ATRIGEL THE MAGICAL TOOL. Int J Current Pharm Res. 2018;10(2):38–42. ArticleGoogle Scholar
- Kempe S, Mäder K. In situ forming implants - an attractive formulation principle for parenteral depot formulations. J Control Release. 2012;161(2):668–79. ArticleCASPubMedGoogle Scholar
- Wright JC, Burgess DJ. Long acting injections and implants. In Chapter 7 (Lipophilic) Oily Solutions and Suspensions. New York, NY: Springer; 2012. BookGoogle Scholar
- Stella VJ, Charman WN, Naringrekar VH. Prodrugs. Do they have advantages in clinical practice? Drugs. 1985;29(5):455–73. ArticleCASPubMedGoogle Scholar
- Stella V, Borchardt R, Hageman M, Oliyai R, Maag H, Tilley J. Prodrugs: challenges and rewards: Springer Sci Bus Med. 2007.
- Information. NCfB. PubChem Compound Summary for CID 5281881, Flupentixol. https://pubchem.ncbi.nlm.nih.gov/compound/cis-Flupentixol. In.
- Procyshyn RM, Lamoure JW, Katzman MA, Skinner PL, Sherman SE. Need for Bioequivalence Standards that Reflect the Clinical Importance of the Complex Pharmacokinetics of Paliperidone Palmitate Long-Acting Injectable Suspension. J Pharm Pharm Sci. 2020;22(1):548–66. Google Scholar
- Chamanza R, Darville N, van Heerden M, De Jonghe S. Comparison of the Local Tolerability to 5 Long-acting Drug Nanosuspensions with Different Stabilizing Excipients, Following a Single Intramuscular Administration in the Rat. Toxicol Pathol. 2017;46(1):85–100. ArticlePubMedGoogle Scholar
- Nguyen V, Bevernage J, Darville N, Tistaert C, Van Bocxlaer J, Rossenu S, Vermeulen A. Linking In Vitro Intrinsic Dissolution Rate and Thermodynamic Solubility with Pharmacokinetic Profiles of Bedaquiline Long-Acting Aqueous Microsuspensions in Rats. Mol Pharm. 2021;18(3):952–65. ArticleCASPubMedGoogle Scholar
- Drugbank, https://go.drugbank.com/drugs. 2022 August 19th, 2022.
- Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–72. ArticleCASPubMedPubMed CentralGoogle Scholar
- Herrero-Vanrell R, Bravo-Osuna I, Andrés-Guerrero V, Vicario-de-la-Torre M, Molina-Martínez IT. The potential of using biodegradable microspheres in retinal diseases and other intraocular pathologies. Prog Retin Eye Res. 2014;42:27–43. ArticleCASPubMedGoogle Scholar
- Cilurzo F, Selmin F, Minghetti P, Adami M, Bertoni E, Lauria S, Montanari L. Injectability evaluation: an open issue. AAPS PharmSciTech. 2011;12(2):604–9. ArticlePubMedPubMed CentralGoogle Scholar
- Rodrigues EB, Grumann A Jr, Penha FM, Shiroma H, Rossi E, Meyer CH, Stefano V, Maia M, Magalhaes O Jr, Farah ME. Effect of needle type and injection technique on pain level and vitreal reflux in intravitreal injection. J Ocul Pharmacol Ther. 2011;27(2):197–203. ArticleCASPubMedGoogle Scholar
- Berteau C, Filipe-Santos O, Wang T, Rojas HE, Granger C, Schwarzenbach F. Evaluation of the impact of viscosity, injection volume, and injection flow rate on subcutaneous injection tolerance. Med Devices (Auckl). 2015;8:473–84. PubMedGoogle Scholar
- Puthli S, Vavia PR. Stability studies of microparticulate system with piroxicam as model drug. AAPS PharmSciTech. 2009;10(3):872–80. ArticleCASPubMedPubMed CentralGoogle Scholar
- Chitnis GD, Verma MKS, Lamazouade J, Gonzalez-Andrades M, Yang K, Dergham A, Jones PA, Mead BE, Cruzat A, Tong Z, Martyn K, Solanki A, Landon-Brace N, Karp JM. A resistance-sensing mechanical injector for the precise delivery of liquids to target tissue. Nat Biomed Eng. 2019;3(8):621–31. ArticleCASPubMedPubMed CentralGoogle Scholar
- Sintzel MB, Merkli A, Tabatabay C, Gurny R. Influence of irradiation sterilization on polymers used as drug carriers—a review. Drug Dev Ind Pharm. 1997;23(9):857–78. ArticleCASGoogle Scholar
- Reinhold SE, Desai KG, Zhang L, Olsen KF, Schwendeman SP. Self-healing microencapsulation of biomacromolecules without organic solvents. Angew Chem Int Ed Engl. 2012;51(43):10800–3. ArticleCASPubMedGoogle Scholar
- Jiang W, Gupta RK, Deshpande MC, Schwendeman SP. Biodegradable poly(lactic-co-glycolic acid) microparticles for injectable delivery of vaccine antigens. Adv Drug Deliv Rev. 2005;57(3):391–410. ArticleCASPubMedGoogle Scholar
- Carrascosa C, Espejo L, Torrado S, Torrado JJ. Effect of gamma-sterilization process on PLGA microspheres loaded with insulin-like growth factor-I (IGF-I). J Biomater Appl. 2003;18(2):95–108. ArticleCASPubMedGoogle Scholar
- Loh ZH, Samanta AK, Sia Heng PW. Overview of milling techniques for improving the solubility of poorly water-soluble drugs. Asian J Pharm Sci. 2015;10(4):255–74. ArticleGoogle Scholar
- Möschwitzer JP. Drug nanocrystals in the commercial pharmaceutical development process. Int J Pharm. 2013;453(1):142–56. ArticlePubMedGoogle Scholar
- EMA/586324/2020, Vocabria Assessment Report, https://www.ema.europa.eu/en/documents/assessment-report/vocabria-epar-public-assessment-report_en.pdf. EMA. 2021 November 17, 2021.
- Cabana A, Rouge B, Markland P. Microspheres for sustained release of octreotide acetate, Patent WO2011112576A1. In.; 2011.
- Fineman M, MacConell L, Taylor K. Methods for treating diabetes and reducing body weight, Patent US8329648B2. In.; 2006.
- Lyons L, Wright S. Device and method for producing Microparticles, Patent AK169/1694, CA2390284A1. In.; 2008.
- Ehrich E. Naltrexone long acting formulations and methods of use, Patent US7919499B2. 2005.
- Dunn R. Sustained Release Polymer, Patent US8470359B2. In.; 2006.
- Ducrey B, P. G, Curdy C, Bardet M, Porchet H, Lundstrom E, Heimgartner F. Slow release pharmaceutical composition made of microgranules, Patent US10166181B2. In.; 2008.
- Bodick N, Blanks RC, Kumar A, Clayman MD, Moran M. Corticosteroids for the treament of joint pain. Patent US8828440B2. In.; 2011.
- Johnson AR, Forster SP, White D, Terife G, Lowinger M, Teller RS, Barrett SE. Drug eluting implants in pharmaceutical development and clinical practice. Expert Opin Drug Deliv. 2021;18(5):577–93. ArticleCASPubMedGoogle Scholar
- Knox ED, Stimmel GL. Clinical review of a long-acting, injectable formulation of risperidone. Clin Ther. 2004;26(12):1994–2002. ArticleCASPubMedGoogle Scholar
- Yáñez JA, Remsberg CM, Sayre CL, Forrest ML, Davies NM. Flip-flop pharmacokinetics–delivering a reversal of disposition: challenges and opportunities during drug development. Ther Deliv. 2011;2:643–72. ArticlePubMedGoogle Scholar
- Darville N, van Heerden M, Vynckier A, De Meulder M, Sterkens P, Annaert P, Van den Mooter G. Intramuscular administration of paliperidone palmitate extended-release injectable microsuspension induces a subclinical inflammatory reaction modulating the pharmacokinetics in rats. J Pharm Sci. 2014;103:2072–87. ArticleCASPubMedGoogle Scholar
- Chamanza R, Darville N, van Heerden M, De Jonghe S. Comparison of the local tolerability to 5 long-acting drug nanosuspensions with different stabilizing excipients, following a single intramuscular administration in the rat. Toxicol Pathol. 2018;46:85–100. ArticleCASPubMedGoogle Scholar
- Zuidema J, Kadir F, Titulaer HAC, Oussoren C. Release and absorption rates of intramuscularly and subcutaneously injected pharmaceuticals (II). Int J Pharm. 1994;105:189–207. ArticleCASGoogle Scholar
- Medlicott NJ, Waldron NA, Foster TP. Sustained release veterinary parenteral products. Adv Drug Deliv Rev. 2004;56(10):1345–65. ArticleCASPubMedGoogle Scholar
- McLennan DN, Porter CJH, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discovery Today. 2005;2:89–96. ArticleCASPubMedGoogle Scholar
- Spanarello S, Ferla TL. The pharmacokinetics of long-acting antipsychotic medications. Curr Clin Pharmacol. 2014;9:310–7. ArticleCASPubMedGoogle Scholar
- Shah JC, Hong J. Model for Long Acting Injectables (Depot Formulation) Based on Pharmacokinetics and Physical Chemical Properties. Aaps j. 2022;24(3):44. ArticleCASPubMedGoogle Scholar
- Nielsen LL, Young AA, Parkes DG. Pharmacology of exenatide (synthetic exendin-4): a potential therapeutic for improved glycemic control of type 2 diabetes. Regul Pept. 2004;117(2):77–88. ArticleCASPubMedGoogle Scholar
- Cauvin AJ, Peters C, Brennan F. Advantages and limitations of commonly used nonhuman primate species in research and development of biopharmaceuticals. In. The Nonhuman Primate in Nonclinical Drug Development and Safety Assessment; 2015. p. 379–395.
- Darville N, Saarinen J, Isomäki A, Khriachtchev L, Cleeren D, Sterkens M, van Heerden M, Annaert P, Peltonen L, Santos HA, Strachan CJ, Van den Mooter G. Multimodal non-linear optical imaging for the investigation of drug nano-/microcrystal-cell interactions. Eur J Pharm Biopharm. 2015;96:338–48. ArticleCASPubMedGoogle Scholar
- Owen A, Rannard S. Strengths, weaknesses, opportunities and challenges for long acting injectable therapies: Insights for applications in HIV therapy. Adv Drug Del Rev. 2016;103:144–56. ArticleCASGoogle Scholar
- Gray V, Cady S, Curran D, DeMuth J, Eradiri O, Hussain M, Krämer J, Shabushnig J, Stippler E. In Vitro Release Test Methods for Drug Formulations for Parenteral Applications. In.Dissolution Technol 2018. p. 8 - 13.
- USP-NF. USP In Vitro Release Test Methods for Parenteral Drug Preparations. https://doi.usp.org/USPNF/USPNF_M5433_02_01.html. USP. 2022 August, 19th.
- S. C, K. BA. Current Updates on In-vitro Drug Release Testing of Long-Acting Injectables, https://www.americanpharmaceuticalreview.com/Featured-Articles/584542-Current-Updates-on-In-vitro-Drug-Release-Testing-of-Long-Acting-Injectables/. 2022 August 19th, 2022.
- Smith WC, Bae J, Zhang Y, Qin B, Wang Y, Kozak D, Ashraf M, Xu X. Impact of particle flocculation on the dissolution and bioavailability of injectable suspensions. Int J Pharm. 2021;604: 120767. ArticleCASPubMedGoogle Scholar
- Kim Y, Park EJ, Kim TW, Na DH. Recent process in drug release testing methods of biopolymeric particulate system. Pharmaceutics. 2021;13(8):1313. ArticleCASPubMedPubMed CentralGoogle Scholar
- Dadhaniya TM, Sharma OP, Gohel MC, Mehta PJ. Current approaches for in vitro drug release study of long acting parenteral formulations. Curr Drug Deliv. 2015;12:256–70. ArticleCASPubMedGoogle Scholar
- Andhariya JV, Shen J, Choi S, Wang Y, Zou Y, Burgess DJ. Development of in vitro-in vivo correlation of parenteral naltrexone loaded polymeric microspheres. J Control Release. 2017;255:27–35. ArticleCASPubMedGoogle Scholar
- Simon A, de Almeida Borges VR, Cabral LM, de Sousa VP. Development and validation of a discriminative dissolution test for betamethasone sodium phosphate and betamethasone dipropionate intramuscular injectable suspension. AAPS PharmSciTech. 2013;14(1):425–34. ArticleCASPubMedPubMed CentralGoogle Scholar
- Marques MRC, Loebenberg R, Almukainzi M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolut Technol. 2011;18:15–28. ArticleCASGoogle Scholar
- Barrett SE, Teller RS, Forster SP, Li L, Mackey MA, Skomski D, Yang Z, Fillgrove KL, Doto GJ, Wood SL, Lebron J, Grobler JA, Sanchez RI, Liu Z, Lu B, Niu T, Sun L, Gindy ME. Extended-Duration MK-8591-Eluting Implant as a Candidate for HIV Treatment and Prevention. Antimicrob Agents Chemother. 2018;62(10).
- D’Souza SS, DeLuca PP. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm Res. 2006;23:460–74. ArticleCASPubMedGoogle Scholar
- Shen J, Burgess D. Accelerated in vitro release testing methods for extended release parenteral dosage forms. J Pharm Pharmacol. 2012;64:986–96. ArticleCASPubMedPubMed CentralGoogle Scholar
- Garner J, Skidmore S, Park H, Park K, Choi S, Wang Y. Beyond Q1/Q2: The Impact of Manufacturing Conditions and Test Methods on Drug Release From PLGA-Based Microparticle Depot Formulations. J Pharm Sci. 2018;107(1):353–61. ArticleCASPubMedGoogle Scholar
- Forrest WP, Reuter KG, Shah V, Kazakevich I, Heslinga M, Dudhat S, Patel S, Neri C, Mao Y. USP Apparatus 4: a Valuable In Vitro Tool to Enable Formulation Development of Long-Acting Parenteral (LAP) Nanosuspension Formulations of Poorly Water-Soluble Compounds. AAPS PharmSciTech. 2018;19(1):413–24. ArticleCASPubMedGoogle Scholar
- Bhardwaj U, Burgess DJ. A novel USP apparatus 4 based release testing method for dispersed systems. Int J Pharm. 2010;388(1–2):287–94. ArticleCASPubMedGoogle Scholar
- Zolnik BS, Raton J-L, Burgess DJ. Application of USP Apparatus 4 and In Situ Fiber Optic Analysis to Microsphere Release Testing. In. Dissolution Technol. 2005. p. 11–14.
- Chidambaram N, Burgess DJ. A novel in vitro release method for submicron-sized dispersed systems. AAPS PharmSci. 1999;1(3):32–40. ArticlePubMed CentralGoogle Scholar
- Liu Y, Sun Y, Sun J, Zhao N, Sun M, He Z. Preparation and in vitro/in vivo evaluation of sustained-release venlafaxine hydrochloride pellets. Int J Pharm. 2012;426(1–2):21–8. ArticleCASPubMedGoogle Scholar
- Savaşer A, Esim O, Kurbanoglu S, Ozkan S, Ozkan Y. Current perspectives on drug release studies from polymeric nanoparticles. In; 2018. p. 101–145.
- Kinnunen HM, Sharma V, Contreras-Rojas LR, Yu Y, Alleman C, Sreedhara A, Fischer S, Khawli L, Yohe ST, Bumbaca D, Patapoff TW, Daugherty AL, Mrsny RJ. A novel in vitro method to model the fate of subcutaneously administered biopharmaceuticals and associated formulation components. J Control Release. 2015;214:94–102. ArticleCASPubMedGoogle Scholar
- Rawat A, Stippler E, Shah VP, Burgess DJ. Validation of USP apparatus 4 method for microsphere in vitro release testing using Risperdal Consta. Int J Pharm. 2011;420(2):198–205. ArticleCASPubMedGoogle Scholar
- Zolnik BS, Leary PE, Burgess DJ. Elevated temperature accelerated release testing of PLGA microspheres. J Control Release. 2006;112(3):293–300. ArticleCASPubMedGoogle Scholar
- Shen J, Burgess DJ. Accelerated in-vitro release testing methods for extended-release parenteral dosage forms. J Pharm Pharmacol. 2012;64(7):986–96. ArticleCASPubMedPubMed CentralGoogle Scholar
- Andhariya JV, Burgess DJ. Recent advances in testing of microsphere drug delivery systems. Expert Opin Drug Deliv. 2016;13(4):593–608. ArticleCASPubMedGoogle Scholar
- Kang J, Schwendeman SP. Pore Closing and Opening in Biodegradable Polymers and Their Effect on the Controlled Release of Proteins. Mol Pharm. 2007;4(1):104–18. ArticleCASPubMedGoogle Scholar
- Fv Burkersroda, Schedl L, Göpferich A. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 2002;23(21):4221–31. ArticleGoogle Scholar
- Guse C, Koennings S, Kreye F, Siepmann F, Göpferich A, Siepmann J. Drug release from lipid-based implants: elucidation of the underlying mass transport mechanisms. Int J Pharm. 2006;314(2):137–44. ArticleCASPubMedGoogle Scholar
- Koennings S, Berié A, Tessmar J, Blunk T, Goepferich A. Influence of wettability and surface activity on release behavior of hydrophilic substances from lipid matrices. J Control Release. 2007;119(2):173–81. ArticleCASPubMedGoogle Scholar
- Kamberi M, Nayak S, Myo-Min K, Carter TP, Hancock L, Feder D. A novel accelerated in vitro release method for biodegradable coating of drug eluting stents: Insight to the drug release mechanisms. Eur J Pharm Sci. 2009;37(3–4):217–22. ArticleCASPubMedGoogle Scholar
- Jucker B, Alsaid H, Rambo M, Lenhard SC, Hoang B, Xie F, Groseclose MR, Castellino S, Damian V, Bowers G, Gupta M. Multimodal imaging approach to examine biodistribution kinetics of Cabotegravir (GSK1265744) long acting parenteral formulation in rat. J Controlled Release. 2017;268:102–12. ArticleCASGoogle Scholar
- Jucker B, Fuchs EJ, Lee S, Damian V, Galette P, Janiczek R, Macura KJ, Jacobs MA, Weld ED, Solaiyappan M, D'Amico R, Shaik JS, Bakshi K, Han K, Ford S, Margolis D, Spreen W, Gupta MK, Hendrix CW, Patel P. Multiparametric magnetic resonance imaging to characterize cabotegravir long-acting formulation depot kinetics in healthy adult volunteers. Br J Clin Pharmacol. 2021:1 - 12.
- Rajoli RKR, Back DJ, Rannard S, Freel Meyers CL, Flexner C, Owen A, Siccardi M. Physiologically Based Pharmacokinetic Modelling to Inform Development of Intramuscular Long-Acting Nanoformulations for HIV. Clin Pharmacokinet. 2015;54(6):639–50. ArticleCASPubMedPubMed CentralGoogle Scholar
- Rajoli RKR, Podany AT, Moss DM, Swindells S, Flexner C, Owen A, Siccardi M. Modelling the long-acting administration of anti-tuberculosis agents using PBPK: a proof of concept study. Int J Tuberc Lung Dis. 2018;22(8):937–44. ArticleCASPubMedGoogle Scholar
- Rajoli RKR, Flexner C, Chiong J, Owen A, Donnelly RF, Larrañeta E, Siccardi M. Modelling the intradermal delivery of microneedle array patches for long-acting antiretrovirals using PBPK. Eur J Pharm Biopharm. 2019;144:101–9. ArticleCASPubMedPubMed CentralGoogle Scholar
- Rajoli RKR, Curley P, Chiong J, Back D, Flexner C, Owen A, Siccardi M. Predicting Drug-Drug Interactions Between Rifampicin and Long-Acting Cabotegravir and Rilpivirine Using Physiologically Based Pharmacokinetic Modeling. J Infect Dis. 2019;219(11):1735–42. ArticleCASPubMedGoogle Scholar
- Perazzolo S, Shireman LM, Koehn J, McConnachie LA, Kraft JC, Shen DD, Ho RJY. Three HIV Drugs, Atazanavir, Ritonavir, and Tenofovir, Coformulated in Drug-Combination Nanoparticles Exhibit Long-Acting and Lymphocyte-Targeting Properties in Nonhuman Primates. J Pharm Sci. 2018;107(12):3153–62. ArticleCASPubMedPubMed CentralGoogle Scholar
- Anand O, Yu LX, Conner DP, Davit BM. Dissolution testing for generic drugs: an FDA perspective. Aaps j. 2011;13(3):328–35. ArticlePubMedPubMed CentralGoogle Scholar
- Shen J, Choi S, Qu W, Wang Y, Burgess DJ. In vitro-in vivo correlation of parenteral risperidone polymeric microspheres. J Control Release. 2015;218:2–12. ArticleCASPubMedPubMed CentralGoogle Scholar
- Rudd ND, Reibarkh M, Fang R, Mittal S, Walsh PL, Brunskill APJ, Forrest WP. Interpreting In Vitro Release Performance from Long-Acting Parenteral Nanosuspensions Using USP-4 Dissolution and Spectroscopic Techniques. Mol Pharm. 2020;17(5):1734–47. ArticleCASPubMedGoogle Scholar
- FDA. FY2018 Regulatory Science Report: Long Acting Injectables and Implants, https://www.fda.gov/media/129010/download. FDA. 2022 August, 19th.
- Matthews RP, Patel M, Barrett SE, Haspeslagh L, Reynders T, Zhang S, Rottey S, Goodey A, Vargo RC, Grobler JA, Stoch SA, Iwamoto M. Safety and pharmacokinetics of islatravir subdermal implant for HIV-1 pre-exposure prophylaxis: a randomized, placebo-controlled phase 1 trial. Nat Med. 2021;27(10):1712–7. ArticleCASPubMedGoogle Scholar
- Anderson JM, Rodriguez A, Chang DT. Foreign body reaction to biomaterials. In.Seminars in immunology: Elsevier; 2008. p. 86–100.
- Zuidema J, Kadir F, Titulaer HAC, Oussoren C. Release and absorption rates of intramuscularly and subcutaneously injected pharmaceuticals (II). Int J Pharm. 1994;105(3):189–207. ArticleCASGoogle Scholar
- Samtani MN, Vermeulen A, Stuyckens K. Population pharmacokinetics of intramuscular paliperidone palmitate in patients with schizophrenia: a novel once-monthly, long-acting formulation of an atypical antipsychotic. Clin Pharmacokinet. 2009;48(9):585–600. ArticleCASPubMedGoogle Scholar
- Medlicott NJ, Waldron NA, Foster TP. Sustained release veterinary parenteral products. Adv Drug Deliv Rev. 2004;56(10):1345–65. ArticleCASPubMedGoogle Scholar
- McLennan DN, Porter CJ, Charman SA. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005;2(1):89–96. ArticleCASPubMedGoogle Scholar
- Lebre F, Sridharan R, Sawkins MJ, Kelly DJ, O’Brien FJ, Lavelle EC. The shape and size of hydroxyapatite particles dictate inflammatory responses following implantation. Sci Rep. 2017;7(1):2922. ArticlePubMedPubMed CentralGoogle Scholar
- Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discovery. 2004;3(9):785–96. ArticleCASPubMedGoogle Scholar
- The United States Pharmacopeia. The National formulary. 2000.
- Grim M, Rerabkova L, Carlson BM. A Test for Muscle Lesions and their Regeneration Following Intramuscular Drug Application. Toxicol Pathol. 1988;16(4):432–42. ArticleCASPubMedGoogle Scholar
- EMA/737723/2013, ABILIFY MAINTENA Assessment Report ,https://www.ema.europa.eu/en/documents/assessment-report/abilify-maintena-epar-public-assessment-report_en.pdf. EMA. 2022 March, 7, 2022.
- Sun Y, Lu X, Gai Y, Sha C, Leng G, Yang X, Liu W. LC-MS/MS method for the determination of the prodrug aripiprazole lauroxil and its three metabolites in plasma and its application to in vitro biotransformation and animal pharmacokinetic studies. J Chromatogr B. 2018;1081–1082:67–75. ArticleGoogle Scholar
- Buss N, Ryan P, Baughman T, Roy D, Patterson C, Gordon C, Dixit R. Nonclinical safety and pharmacokinetics of Miglyol 812: A medium chain triglyceride in exenatide once weekly suspension. J Appl Toxicol. 2018;38(10):1293–301. ArticleCASPubMedGoogle Scholar
- Li Y, Vaughan KL, Tweedie D, Jung J, Kim HK, Choi H-I, Kim DS, Mattison JA, Greig NH. Pharmacokinetics of Exenatide in nonhuman primates following its administration in the form of sustained-release PT320 and Bydureon. Sci Rep. 2019;9(1):17208. ArticlePubMedPubMed CentralGoogle Scholar
- Gedulin BR, Smith P, Prickett KS, Tryon M, Barnhill S, Reynolds J, Nielsen LL, Parkes DG, Young AA. Dose–response for glycaemic and metabolic changes 28 days after single injection of long-acting release exenatide in diabetic fatty Zucker rats. Diabetologia. 2005;48(7):1380–5. ArticleCASPubMedGoogle Scholar
- Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2 Suppl 1):25–31. ArticlePubMedGoogle Scholar
- Okada H, Inoue Y, Heya T, Ueno H, Ogawa Y, Toguchi H. Pharmacokinetics of Once-a-Month Injectable Microspheres of Leuprolide Acetate. Pharm Res. 1991;8(6):787–91. ArticleCASPubMedGoogle Scholar
- Okada H, Doken Y, Ogawa Y, Toguchi H. Preparation of Three-Month Depot Injectable Microspheres of Leuprorelin Acetate Using Biodegradable Polymers. Pharm Res. 1994;11(8):1143–7. ArticleCASPubMedGoogle Scholar
- FDA, Paliperidone Palmitate, NDA 22–264, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2009/022264s000clinpharmr.pdf. FDA. 2022 March 7, 2022. Available from: EMA/586324/2020, Vocabria Assessment Report, https://www.ema.europa.eu/en/documents/assessment-report/vocabria-epar-public-assessment-report_en.pdf.
- Ereshefsky L, Mannaert E. Pharmacokinetic profile and clinical efficacy of long-acting risperidone: potential benefits of combining an atypical antipsychotic and a new delivery system. Drugs R D. 2005;6(3):129–37. ArticleCASPubMedGoogle Scholar
- Murty SB, Wei Q, Thanoo BC, DeLuca PP. In vivo release kinetics of octreotide acetate from experimental polymeric microsphere formulations using oil/water and oil/oil processes. AAPS PharmSciTech. 2004;5(3):90–9. ArticlePubMed CentralGoogle Scholar
- Comets E, Mentré F, Kawai R, Nimmerfall F, Marbach P, Vonderscher J. Modeling the kinetics of release of octreotide from long-acting formulations injected intramuscularly in rabbits. J Pharm Sci. 2000;89(9):1123–33. ArticleCASPubMedGoogle Scholar
- Comets E, Mentré F, Nimmerfall F, Kawai R, Mueller I, Marbach P, Vonderscher J. Nonparametric analysis of the absorption profile of octreotide in rabbits from long-acting release formulation OncoLAR. J Control Release. 1999;59(2):197–205. ArticleCASPubMedGoogle Scholar
- T. O, Gelder M, O’Boyle E. Biochronomer™ technology and the development of APF530, a sustained release formulation of granisetron. J Exp Pharmacol. 2014;6:15–21. https://doi.org/10.2147/JEP.S68880. ArticleGoogle Scholar
- Weinbauer GF, Jackwerth B, Yoon YD, Behre HM, Yeung CH, Nieschlag E. Pharmacokinetics and pharmacodynamics of testosterone enanthate and dihydrotestosterone enanthate in non-human primates. Acta Endocrinol (Copenh). 1990;122(4):432–42. CASPubMedGoogle Scholar
- Kane FE, Green KE. Ocular Pharmacokinetics of Fluocinolone Acetonide Following Iluvien Implantation in the Vitreous Humor of Rabbits. J Ocul Pharmacol Ther. 2015;31(1):11–6. ArticleCASPubMedPubMed CentralGoogle Scholar
- Johnson JT, Everly AG, Kpakima FEF, Detke HC. Postmortem redistribution of olanzapine following intramuscular administration of olanzapine pamoate in dogs. Forensic Sci Int. 2015;257:353–8. ArticleCASPubMedGoogle Scholar
Author information
- Venkat R. Koganti and Stephanie E. Barrett are Team leads of the IQ consortium “Long-Acting Injectables” Working Group.
Authors and Affiliations
- Sunovion Pharmaceuticals, Marlborough, MA, 01752, USA Andrea Bauer
- UCB Pharma SA, Braine-l’Alleud, Belgium Philippe Berben
- Bristol Myers Squibb, New Brunswick, NJ, 08901, USA Sudhir S. Chakravarthi & Venkat R. Koganti
- GlaxoSmithKline, Collegeville, PA, 19426, USA Sayantan Chattorraj, Christopher Morrison, Deepak Mundhra & Pratik Saha
- Eli Lilly and Company, Indianapolis, IN, USA Ashish Garg & Shweta Urva
- Biogen, Cambridge, MA, 02142, USA Betty Gourdon
- Merck & Co., Inc., Rahway, NJ, 07065, USA Tycho Heimbach, Ryan Vargo & Stephanie E. Barrett
- AbbVie Inc., North Chicago, IL, 60064, USA Ye Huang & Yi Shi
- Gilead Sciences, Foster City, CA, 94404, USA Ramesh Palaparthy & Naveed A. Shaik
- Janssen R&D, a Division of Janssen Pharmaceutica NV, Beerse, Belgium Maxime Siemons
- Takeda Development Center Americas, Inc., Cambridge, MA, 02139, USA Sara Shum
- Boehringer Ingelheim Pharmaceuticals, Inc., Ridgefield, CT, USA Naveen K. Thakral
- Andrea Bauer









